*Currently, this product is available in Japan only*
Mycoplasma pneumoniae nucleic acid kit

Features
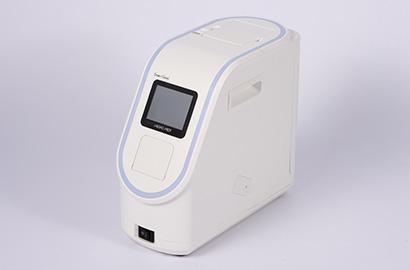
・Dedicated reagent for "Fully automated gene analysis instrument "
・Rapid detection- result of Mycoplasma pneumoniae nucleic acid test is available in just 30~50 minutes from the time of dropping sample
・No complicated preparation required- can be tested at clinics
・Detects all Mycoplasma pneumoniae strains including 23S rRNA mutant strains
Principle
- Extraction
- Capture Mycoplasma pneumoniae DNA by unique extraction method using membrane and silica particles
- Amplification
- Amplify mycoplasma 23s rRNA gene by PCR (amplify all Mycoplasma pneumoniae DNA including macrolide-resistant Mycoplasma pneumoniae)
- Detection
- QProbe method- real-time detection detects Mycoplasma pneumoniae DNA
Correlation
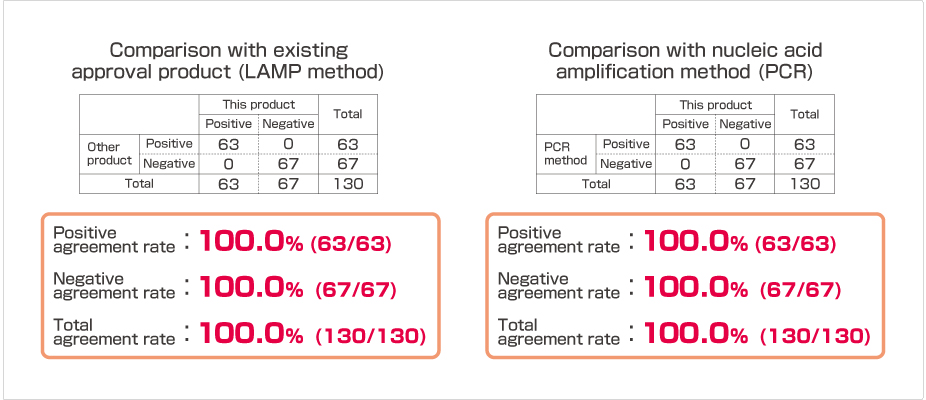
Product Overview
| Specimen | Pharyngeal swab specimen |
|---|---|
| Reaction time | Approximately 30~50 minute (from the time of dropping sample) |
Kit Component
Test cartridge
| Product No. | 50110 |
|---|---|
| Product name | Test cartridge |
| Package | 5 tests/kit |
| Contents | ・Test cartridge -5 tests |
| Expiry | 18 months |
| Storage | 2~8℃ |
Specimen collection set
| Product No. | 50120 |
|---|---|
| Product name | Specimen collection set |
| Package | 10 tests/set |
| Contents | ・Extraction reagent solution vial-0.55mL×10 vials |
| Accessories | Swab (for pharyngeal swab specimen)-10 pieces Filter (for extraction reagent solution vial)-10 pieces Filter cap-10 pieces Name label-1 sheet |
| Expiry | 18 months |
| Storage | 2~30℃ |
Sold separately
Myco Positive control
| Product No. | 59103 |
|---|---|
| Product Name | Myco Positive control |
| Package | 3 tests/kit |
| Content |
・Positive control - 0.5mL×3 vials ・Filter ・Filter cap |
| Expiry | 24 months |
| Storage | 2 - 8℃ |
Specimen Collection and Sample Preparation
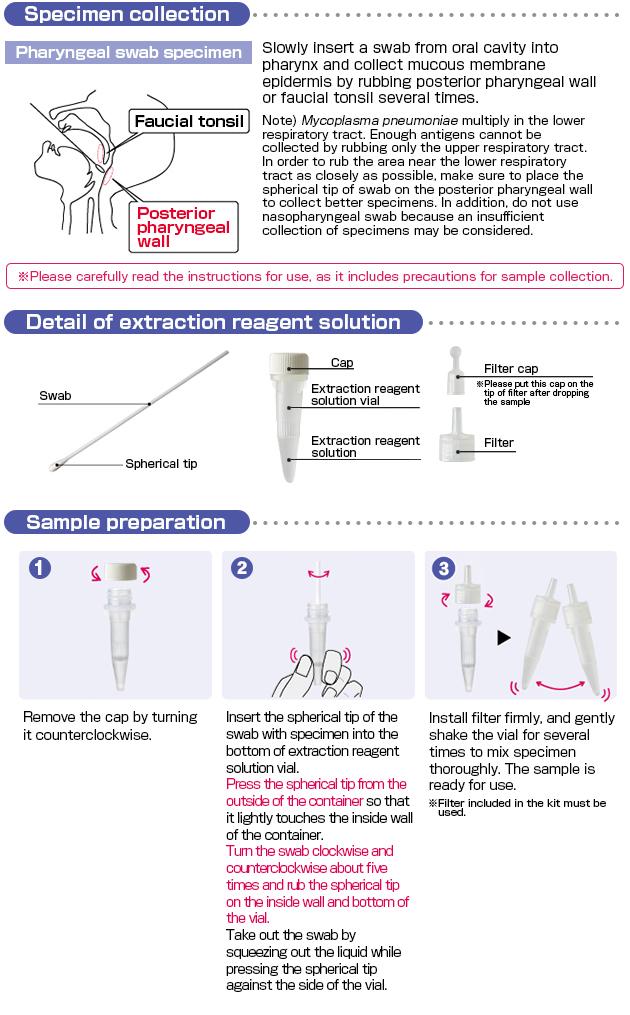
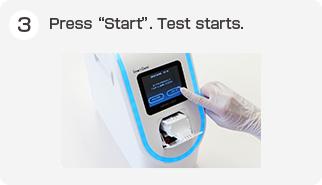
Test Procedure
Simply drop sample to a test cartridge and set to the instrument. Once a cartridge is set, no other operation is needed until the test ends.
Related Product
- Contact Us
-
 Overseas Business Department TEL:+81-942-85-3845 FAX:+81-942-84-5490 Hours:Weekdays 8:30 to 17:30(except Saturdays, Sundays, and Holidays) Contact form
Overseas Business Department TEL:+81-942-85-3845 FAX:+81-942-84-5490 Hours:Weekdays 8:30 to 17:30(except Saturdays, Sundays, and Holidays) Contact form